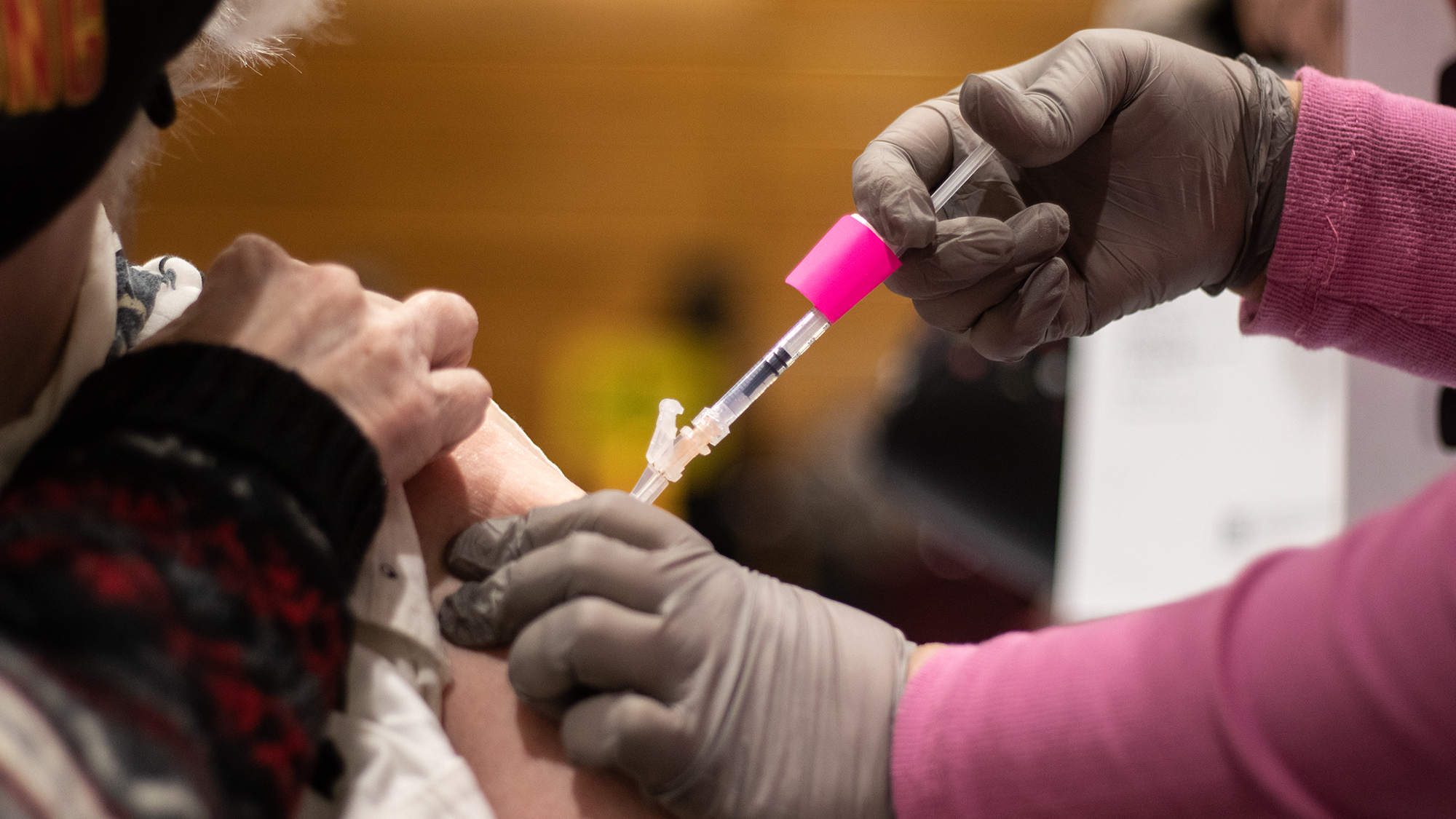
The Maryland Department of Health directed all vaccine providers in the state to pause all Johnson & Johnson vaccinations Tuesday, according to a press release from the department.
The FDA recommended that providers pause administration of these vaccines, after reports of six U.S. cases of a “rare and severe” blood clot in patients who received the Johnson & Johnson vaccine out of more than 6.8 million doses of that vaccine, according to the press release.
[MD opens COVID-19 vaccine appointments to all faculty, students and staff]
The CDC and FDA will further investigate the cases of blood clots out of an abundance of caution, the press release read.
Last month, Prince George’s County received 600 doses of the Johnson & Johnson vaccine.
Providers were directed to store the supply of Johnson & Johnson vaccines to prevent waste.